I designed and prototyped this novel medical device with Preston Lim and Alaina Shumate for the BIOE 141A/B course series (Senior Capstone in Biodesign) in the 2015-2016 academic year. In this class, I learned the Biodesign process for medical technology innovation, specificaly needs finding, needs screening, concept generation, concept screening, and iterative prototyping. My team was given a clinical observation and we identified an unmet clinical need, namely for an efficacious, comfortable, and convenient method to improve venous blood flow in the legs of patients with chronic venous insufficiency. We then conceptualized, designed, and built a functional prototype of a wearable medical device to meet this need. We quantitatively verified efficacy specifications by developing a leg and vein model for real-time digital measurement and display of blood flow response to our device. We have filed a provisional patent application and are proceeding with the IRB approval process for a pilot study to test the efficacy and comfort of our device as compared to the standard of care. We have also submitted our invention to student design competitions.
Executive Summary
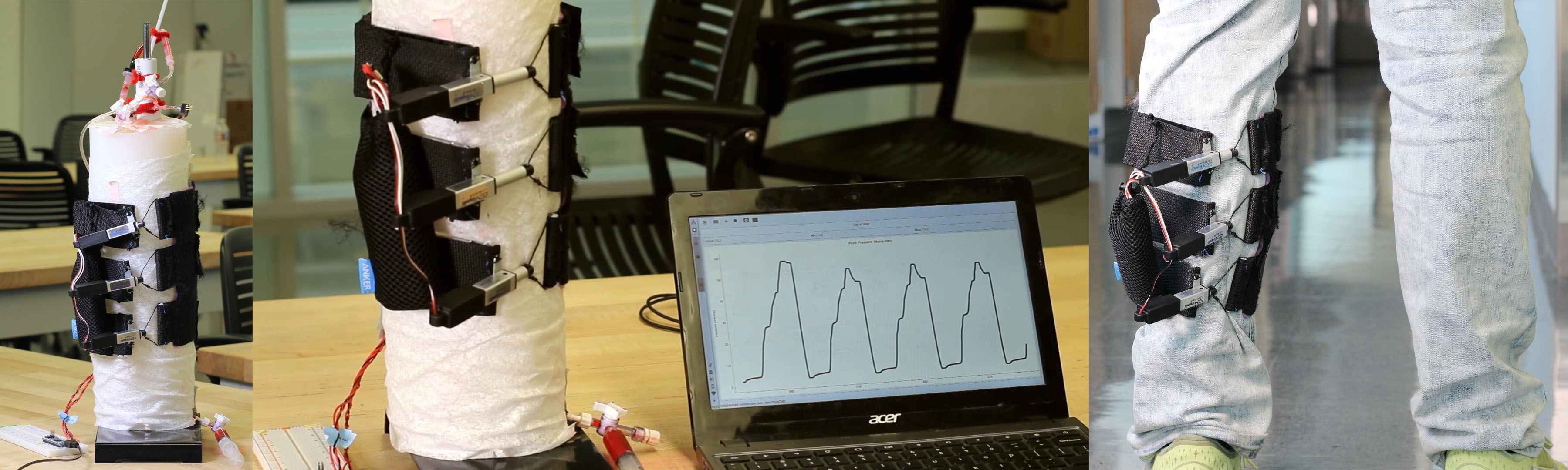
Chronic venous insufficiency (CVI) is the persistent presence of pain, edema, skin changes, or skin ulceration in the lower limbs due to impaired venous return of blood. The standard CVI treatment, compression stockings, is effective but suffers from low patient compliance. There is a need for a more comfortable, convenient, and usable method to efficaciously improve lower extremity venous return for patients with CVI. To meet this need, our team designed the Venous Return Assistance leg sleeve (VERA sleeve), a sequentially contracting leg sleeve. The novel mechanical design features contractile fabric bands, each possessing an actuator to produce sequential contractions around the leg.
Abstract
Chronic venous insufficiency (CVI) is the persistent presence of pain, edema, skin changes, or skin ulceration in the lower limbs due to impaired venous return of blood. CVI has a prevalence estimated at 6% of the US population and presents a significant quality-of-life burden. Severe CVI cases alone cost US healthcare payers an estimated $15 billion per year. The standard CVI treatment, compression stockings, is effective but suffers from low patient compliance due to comfort and usability issues with constriction of the leg and difficulty in putting on the stockings. Thus, there is a need for a comfortable and convenient method to improve lower extremity venous return for patients with chronic venous insufficiency in order to reduce lower extremity pain and swelling. Our team designed the Venous Return Assistance leg sleeve (VERA sleeve), a sequentially contracting leg sleeve to improve venous return in patients with CVI. The design consists of three contractile fabric bands, each possessing a motor-based actuator to produce sequential contractions around the leg. The VERA sleeve runs on a portable rechargeable battery for 10 hours of ambulatory use. When evaluated on a leg model test fixture, the VERA sleeve is able to produce a peak venous pressure of 55 mmHg, comparable to pressures generated by bedside pneumatic sequential compression devices for CVI. These results demonstrate feasibility for a sequentially contracting, ambulatory, outpatient solution. The comfort, convenience, and efficacy of the VERA sleeve has potential to greatly improve quality of life for patients suffering from this burdensome condition.
Full Report
Depending on the results of our design competition submissions, we may post our full project report.
Contributions and Acknowledgements
All members of our team contributed equally to the development of the VERA sleeve. Throughout our project, our team was mentored by Dr. Ross Venook and Dr. Kara Rogers for logistics and project planning. We are proceeding with the IRB approval process for a pilot study with the guidance of Dr. Bryant Lin. We filed our provisional patent application through the Stanford Office of Technology Licensing with the names of all three team members on the application.